This seminar was developed by Keegan Korthauer
This seminar focuses on a portion of the upstream preprocessing of RNA-seq: going from aligned reads (BAM files) to count tables that will be used in differential expression analysis. Note that we are skipping the step of going from raw reads (fastq files) to aligned reads (BAM files), as this is outside the scope of this course. Refer to the lecture materials on High-dimensional genomics assays & data for high-level details and resources.
In order to use RNA-Seq data for the purposes of differential expression analysis, we need to obtain an expression value for each gene (or transcript). The digital nature of RNA-seq data allows us to use the number of reads (an integer count) which align to a specific feature (gene or transcript) as its expression value. In this seminar, we will explore how to use BAM (or SAM) files to generate a count table of reads aligning to the human transcriptome.
By the end of this seminar, you should be able to:
- use the
Rsamtoolspackage to sort and index a BAM file - use the
Rsubreadpackage to count the number of reads that align to each gene/transcript - extract raw counts from the output of
featureCounts, and calculate normalized expression units using thecpm()andrpkm()functions inedgeR
Before we begin, we’ll need to load the necessary R packages for this
seminar. It is likely you don’t already have all of these installed on
your machine unless you’ve done a similar analysis in the past. If this
is the case, you’ll need to run this code chunk to install them
(currently set to eval = FALSE):
library(BiocManager)
install(c("Rsamtools", "Rsubread", "RNAseqData.HNRNPC.bam.chr14", "edgeR"))After they are successfully installed, we’ll load them for use in our session.
library(Rsamtools)
library(Rsubread)
library(RNAseqData.HNRNPC.bam.chr14)
library(ggplot2)
theme_set(theme_bw())
library(dplyr)
library(edgeR)
library(testthat)SAM (sequence alignment map) and BAM (the binary version of SAM) files are the preferred output of most alignment tools. SAM and BAM files carry the same information. The only difference is that SAM is human readable, meaning that you can visually inspect each of the reads. You can learn more about the SAM file format here.
The
Rsamtools
package is one of many software tools that have been developed for
working with SAM/BAM alignment files - it is an R implementation of the
widely used command line tool Samtools. The
Rsubread
package is a tool that summarizes SAM/BAM files into count tables - it
is an R implementation of the command line program
Subread which has tools for alignment
and quantification. The tool we’ll use within Subread is the
featureCounts
tool.
Although you could use the standalone command line versions of both of these tools, to make things as convenient as possible, we’ll use the R implementations and run today’s seminar entirely within R.
The alignment BAM file we will be working with was created by aligning raw reads from an RNA-seq experiment of HNRNPC gene knockdown cell line and control HeLa cells (Zarnack et al. 2012). We will specifically work with one of the control HeLa cell samples. HeLa cells are immortalized cervical cancer cells derived taken from cancer patient Henrietta Lacks in 1951. Henrietta Lacks was a 31-year-old African-American mother of five and a patient at Johns Hopkins Hospital. She died from cervical cancer on October 4, 1951. Her cells were taken from her without her knowledge or consent, and the HeLa cell line is now the oldest and most used immortal cell line in scientific research. To read more about Henrietta’s legacy and the ethical issues involved in procuring and using her cells, read The Immortal Life of Henrietta Lacks.
In this tutorial, we are working with an already aligned BAM file, but the raw reads (fastq files) from this experiment are available in the European Nucleotide Archive.
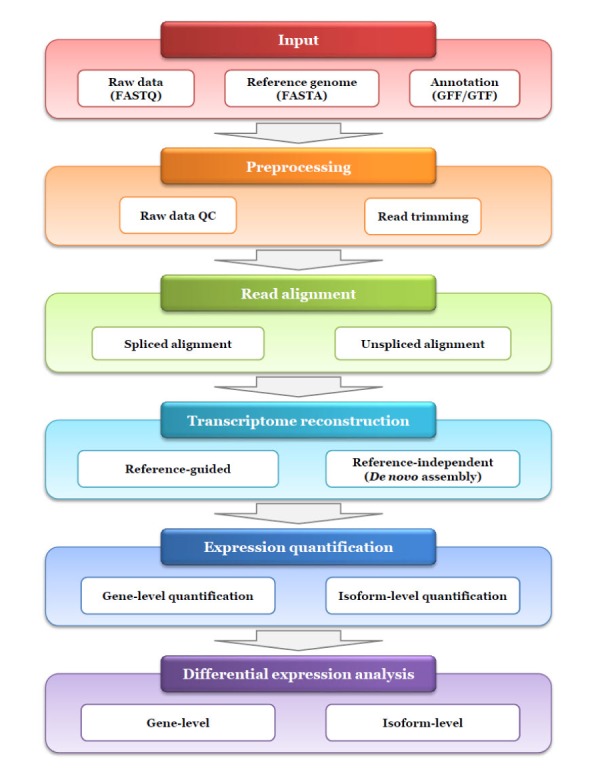
In this tutorial we will only be dealing with a portion of the RNA-seq analysis pipeline. Here is an overview of the entire pipeline (image source: Yang & Kim 2015). Note that this figure is simplistic in its depiction of downstream analysis - differential expression is not the only downstream analysis in typical experiments. For example, we’ll learn about things like gene network and enrichment analyses, clustering, and supervised learning later in the course.
Typical workflow for RNA sequencing data analysis (Yang & Kim 2015)As you can see, there are many steps to go from the raw reads to the aligned bam files (red to light blue). These have been done for us in this example, as we are making use of the aligned data in the Bioconductor package RNAseqData.HNRNPC.bam.chr14. Briefly, this package has preprocessed the data from Zarnack et al. 2012. The reads (paired-end) were aligned to the full human genome (version hg19) with TopHat2. In addition, the data have been subset to only include reads mapped to Chromosome 14. This will reduce the computational time for our tutorial compared to running on the entire genome.
In this tutorial, we are only going to deal with the dark blue box: “Expression Quantification”. Specifically, we are going to learn how to convert a SAM/BAM alignment file to a count table that can be used for differential expression analysis. Along the way, we will learn more about the SAM and BAM files.
The first thing we are going to do is look at the alignment file, so we
can learn more about the structure of BAM/SAM files. Since we’re not
downloading the BAM file directly, but instead using a BAM file included
in the RNAseqData.HNRNPC.bam.chr14 package files, we first need to
find out the file location of where the BAM file is stored. From the
package documentation, we learn that the file names are stored in the
object RNAseqData.HNRNPC.bam.chr14_BAMFILES.
RNAseqData.HNRNPC.bam.chr14_BAMFILES## ERR127306
## "/Library/Frameworks/R.framework/Versions/4.4-arm64/Resources/library/RNAseqData.HNRNPC.bam.chr14/extdata/ERR127306_chr14.bam"
## ERR127307
## "/Library/Frameworks/R.framework/Versions/4.4-arm64/Resources/library/RNAseqData.HNRNPC.bam.chr14/extdata/ERR127307_chr14.bam"
## ERR127308
## "/Library/Frameworks/R.framework/Versions/4.4-arm64/Resources/library/RNAseqData.HNRNPC.bam.chr14/extdata/ERR127308_chr14.bam"
## ERR127309
## "/Library/Frameworks/R.framework/Versions/4.4-arm64/Resources/library/RNAseqData.HNRNPC.bam.chr14/extdata/ERR127309_chr14.bam"
## ERR127302
## "/Library/Frameworks/R.framework/Versions/4.4-arm64/Resources/library/RNAseqData.HNRNPC.bam.chr14/extdata/ERR127302_chr14.bam"
## ERR127303
## "/Library/Frameworks/R.framework/Versions/4.4-arm64/Resources/library/RNAseqData.HNRNPC.bam.chr14/extdata/ERR127303_chr14.bam"
## ERR127304
## "/Library/Frameworks/R.framework/Versions/4.4-arm64/Resources/library/RNAseqData.HNRNPC.bam.chr14/extdata/ERR127304_chr14.bam"
## ERR127305
## "/Library/Frameworks/R.framework/Versions/4.4-arm64/Resources/library/RNAseqData.HNRNPC.bam.chr14/extdata/ERR127305_chr14.bam"
We can see we have 8 BAM files. We’ll use sample ERR127306, which we can determine from the data repository is a control HeLa sample.
bamfile <- RNAseqData.HNRNPC.bam.chr14_BAMFILES[grepl("ERR127306",
RNAseqData.HNRNPC.bam.chr14_BAMFILES)]This file is not human readable, so we are going to convert it into a
SAM file, which we can read. The asSam function converts BAM files to
SAM files. The argument destination = will specify the name of the
newly created SAM file. We’ll save a SAM file to the current working
directory.
asSam(bamfile, destination = "hela")## [1] "hela.sam"
We have created the file “hela.sam”. We’ll take a peek at the top of the header:
cat(system("head hela.sam", intern = TRUE), sep = '\n')## @HD VN:1.0 SO:coordinate
## @SQ SN:chr1 LN:249250621
## @SQ SN:chr10 LN:135534747
## @SQ SN:chr11 LN:135006516
## @SQ SN:chr11_gl000202_random LN:40103
## @SQ SN:chr12 LN:133851895
## @SQ SN:chr13 LN:115169878
## @SQ SN:chr14 LN:107349540
## @SQ SN:chr15 LN:102531392
## @SQ SN:chr16 LN:90354753
This contains information on the format and contents of the file. And at the last few lines of the file, which illustrates some reads and their mapping location, along with lots of extra mysterious-looking information:
cat(system("tail hela.sam", intern = TRUE), sep = '\n')## ERR127306.10965452 163 chr14 106981278 50 72M = 106981361 155 AAGAAACTTACAGTCATGATGGAAAGGGCAGCAAACACGTACCTCTTCACATGGCTGCAGGAGAGAGAAATG +++++++++++*++++++++++++++++*+++++++++++++++++++)++++*+*+&+++++*++++*+)* AS:i:-3 XN:i:0 XM:i:1 XO:i:0 XG:i:0 NM:i:1 MD:Z:69G2 YT:Z:UU NH:i:1
## ERR127306.10965452 83 chr14 106981361 50 72M = 106981278 -155 GGGAGGCCCTTTATATAACCATCAGGTCTTGTGAGAACTCACTCACTAATAGGATAAAAGCATGGAGAGAAC ##+)+++%+*&+++++++*++)+++++++++++++++)+++*+++++++)++*+%++++*++++++)+++++ AS:i:-3 XN:i:0 XM:i:1 XO:i:0 XG:i:0 NM:i:1 MD:Z:68A3 YT:Z:UU NH:i:1
## ERR127306.11919172 163 chr14 106986423 50 72M = 106986528 177 CGGTGGTGTCCTCAGTGGCCCCTGGTGTCCTGAGCATCCCCTGGTGTCCTGAGTGCCCCCTGGTGGTTGTGA ++**+++&+++)++)*++++++()*"''''++(+***)++*&**&)'')&'%&%'&'&''!$""%$"$$!!! AS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:72 YT:Z:UU NH:i:1
## ERR127306.11919172 83 chr14 106986528 50 72M = 106986423 -177 CCTGAGAGCCCCTTGCTGTCCTGAGCACCTCCTGGTGTTCTGAGCGCCCTCTGGTGTTCTGATCACTCTCTG &'&)%*&&)(*****+*+**+*+*++))++*++++++++++++++++**+(+++++++++++++++++++++ AS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:72 YT:Z:UU NH:i:1
## ERR127306.3567919 99 chr14 106989680 50 72M = 106989780 172 CAACTTTTATTTCTTAAACACAAGACATTCCAATGAGAAAGCTGTTCTCAGGTGAGCTGTCGAGCAGGGAGG ++++++++*+++++++*++++++)++++++++++++++++++++++++++++)+&+)++*++*))*+++)+& AS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:72 YT:Z:UU NH:i:1
## ERR127306.3567919 147 chr14 106989780 50 72M = 106989680 -172 GAAATTGCTGAAACTTGAAGACCAAGGCCACCTCTGAGGGGCAGAGATCCACCTATGAGTACATCACATCAG (+++++++&+++)'*+++++*++++++*+*+++++++++++++++++)++++++*+++++++++++++++++ AS:i:-3 XN:i:0 XM:i:1 XO:i:0 XG:i:0 NM:i:1 MD:Z:62G9 YT:Z:UU NH:i:1
## ERR127306.21510817 99 chr14 106994763 50 72M = 106994819 128 CAAAGCTGGATGTGTCTAGTGTTTTTATCAGAACCCACTTTCCGTAATAAGAGCATGTGTGGTTTTGCTGCC ++++++++++++++++++*+)++++++++++++++++++++++++++++*+*++*++)+*+(*++***+**+ AS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:72 YT:Z:UU NH:i:1
## ERR127306.21510817 147 chr14 106994819 50 72M = 106994763 -128 GTGTGGTTTTGCTGCCCTCCAGCACTCTTCTGAAAATATGGAGAGAACTAGGATCCAGGCACATTAATTTTC +%++*+*(**++++**+&++*++)+++++++++++++++++++++++*++++++++++++++++++++++++ AS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:72 YT:Z:UU NH:i:1
## ERR127306.661203 163 chr14 107003080 50 72M = 107003171 163 AAGGAACCCTTGAACTCCCTTGGCGACATGTACTCCTACTAGCACTGTGGCATTATGGTCCTCTGCCTCAAG +++++++++++++++++++++++++++++++++++*++*+*')++++)*+++++(+*()+'*++*+((**') AS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:72 YT:Z:UU NH:i:1
## ERR127306.661203 83 chr14 107003171 50 72M = 107003080 -163 CATGACTTGATGGCTGGAACAAATACATTTAGAGATTTTACCTCCAATACTAGCCTTTGCCATACAGTATTT +(&+*)+)+++++*+++*+*++++*&+&++++++)+'+)(+)+++++++++*++++++++++*+++++++++ AS:i:-3 XN:i:0 XM:i:1 XO:i:0 XG:i:0 NM:i:1 MD:Z:46G25 YT:Z:UU NH:i:1
You can see that each line corresponds to a single read, and each field refers to a different descriptor of the read. For a detailed explanation of what each field means, see this document. Understanding what each field means is not of immediate relevence to the following steps. However, it is always good to have a general understanding of the structure of the files you are working with.
In order to be able to do things like extract reads that align to a certain chromosome, or that map to a certain genomic region, we need to have a BAM file index. The index acts as a table of contents so that tools can quickly jump to parts of the BAM file without having to look through all of the sequences. In this example dataset, one has already been generated for us. But for illustration we’ll review how to create one. To do this, we first have to sort the file. We can do this using the following command to sort our BAM file by chromosome and positions of each read:
sortBam(bamfile, destination="hela_sorted")## [1] "hela_sorted.bam"
This generated a file ‘hela_sorted.bam’ in our current working directory. Once this file has been sorted, we can generate an index file:
indexBam("hela_sorted.bam")## hela_sorted.bam
## "hela_sorted.bam.bai"
Note: you must always sort before indexing a file!
We don’t want to have to look through our entire file line by line.
Let’s instead use some of the handy parsing functions in RSamtools to
view some basic summary info about our file (note these functions can
use the non-human readable BAM file, which is more efficient than the
human-readable SAM file.
First, we create a special reference to the BAM file, which will be passed to the parsing functions (this contains more information than just the path to the BAM file - also points to the location of the BAM index file):
# view basic info of bam
bamFile <- BamFile(bamfile)
bamFile## class: BamFile
## path: /Library/Frameworks/R.framework/Versions/4.4-arm64/.../ERR127306_chr14.bam
## index: /Library/Frameworks/R.framework/Versions/4.4-a.../ERR127306_chr14.bam.bai
## isOpen: FALSE
## yieldSize: NA
## obeyQname: FALSE
## asMates: FALSE
## qnamePrefixEnd: NA
## qnameSuffixStart: NA
We’ll pass this special reference to countBam, which will by default
tell us how many reads and bases are contained in our BAM file.
countBam(bamFile) ## space start end width file records nucleotides
## 1 NA NA NA NA ERR127306_chr14.bam 800484 57634848
We can see we have 800484 reads, which amounts to a total of about 57.6 million nucleotides. From this output, can you determine how long are our reads?
Next, we’ll use the quickBamFlagSummary function to look at the
different ‘flags’ for our reads.
quickBamFlagSummary(bamFile)## group | nb of | nb of | mean / max
## of | records | unique | records per
## records | in group | QNAMEs | unique QNAME
## All records........................ A | 800484 | 393300 | 2.04 / 10
## o template has single segment.... S | 0 | 0 | NA / NA
## o template has multiple segments. M | 800484 | 393300 | 2.04 / 10
## - first segment.............. F | 400242 | 393300 | 1.02 / 5
## - last segment............... L | 400242 | 393300 | 1.02 / 5
## - other segment.............. O | 0 | 0 | NA / NA
##
## Note that (S, M) is a partitioning of A, and (F, L, O) is a partitioning of M.
## Indentation reflects this.
##
## Details for group M:
## o record is mapped.............. M1 | 800484 | 393300 | 2.04 / 10
## - primary alignment......... M2 | 779228 | 389614 | 2 / 2
## - secondary alignment....... M3 | 21256 | 10052 | 2.11 / 8
## o record is unmapped............ M4 | 0 | 0 | NA / NA
##
## Details for group F:
## o record is mapped.............. F1 | 400242 | 393300 | 1.02 / 5
## - primary alignment......... F2 | 389614 | 389614 | 1 / 1
## - secondary alignment....... F3 | 10628 | 10052 | 1.06 / 4
## o record is unmapped............ F4 | 0 | 0 | NA / NA
##
## Details for group L:
## o record is mapped.............. L1 | 400242 | 393300 | 1.02 / 5
## - primary alignment......... L2 | 389614 | 389614 | 1 / 1
## - secondary alignment....... L3 | 10628 | 10052 | 1.06 / 4
## o record is unmapped............ L4 | 0 | 0 | NA / NA
These ‘flags’ can be used to get detailed counts of certain types of reads. As a quick example, we might want to grab the count of reads that on the minus strand, we could do the following:
params <- ScanBamParam(flag = scanBamFlag(isMinusStrand = TRUE))
countBam(bamFile, param = params)## space start end width file records nucleotides
## 1 NA NA NA NA ERR127306_chr14.bam 400242 28817424
There’s a ton more possibilities - explore the documentation of the
RSamtools package (in particular the scanBamFlag and scanBamParam
functions), as well as the meaning of SAM
flags if you’d like to
learn more. These can be really helpful for quality control of our
alignment - to make sure we have what we generally expect (e.g. if we
performed stranded sequencing, we expect that roughly half of the reads
map to each strand). We don’t need to dive too deep into the flags if we
are satisfied with our alignment and just want to proceed to get our
count table, however.
Let’s take stock of where we are: we have a BAM file, a SAM file and a BAI (BAM index) file. It is hard to do much analysis with what we have, because even though we know which chromosome our reads map to, we don’t know which genes (or transcripts) they map to.
A variety of different tools can be used to generate a count table - i.e
a table of how many reads align to each gene. HTSeq, RSEM, and
featureCounts are examples of such programs that are command line
tools. Each tool works optimally with the output of different aligners,
so it is best to read up on how the BAM files you are working with were
processed, and to use the feature counting tools.
Today, we are going to use featureCounts as implemented in the
RSubread package, as the BAM files we are using were generated using
the Tophat2 aligner, which generates BAM files that are compatible with
featureCounts. Because we are trying to associate each read with a
gene ID, we need an alignment file (such as a GTF file) that contains
information on the genomic coordinates of each transcript.
This type of alignment file can be downloaded from a database such as
Gencode, but conveniently, RSubread
has some RefSeq annotations built-in, including for mm10, mm9, hg38, and
hg19. It is critical that you use the same genome version as the
genome version used to align raw reads to your BAM file. Our BAM file
was generated by aligning reads to hg19, so that’s what we’ll use. These
built-in annotations allow for summarizing counts over genes (the
default, and also the option we’ll choose) or exons. Note that external
annotation files can be used for other genomes and to summarize over
transcript/isoform annotations.
Now we’re ready to apply the featureCounts function that will do the
counting of reads over genes. Note that this step can take a while if we
have a large BAM file. We specify isPairedEnd = TRUE since as we
mentioned above our sequencing protocol generated paired end reads. We
also specify strandSpecific = 1 to indicate that our sequencing
protocol was stranded.
genecounts <- featureCounts(bamfile,
annot.inbuilt = "hg19",
isPairedEnd = TRUE,
strandSpecific = 1)## NCBI RefSeq annotation for hg19 (build 37.2) is used.
##
## ========== _____ _ _ ____ _____ ______ _____
## ===== / ____| | | | _ \| __ \| ____| /\ | __ \
## ===== | (___ | | | | |_) | |__) | |__ / \ | | | |
## ==== \___ \| | | | _ <| _ /| __| / /\ \ | | | |
## ==== ____) | |__| | |_) | | \ \| |____ / ____ \| |__| |
## ========== |_____/ \____/|____/|_| \_\______/_/ \_\_____/
## Rsubread 2.20.0
##
## //========================== featureCounts setting ===========================\\
## || ||
## || Input files : 1 BAM file ||
## || ||
## || ERR127306_chr14.bam ||
## || ||
## || Paired-end : yes ||
## || Count read pairs : yes ||
## || Annotation : inbuilt (hg19) ||
## || Dir for temp files : . ||
## || Threads : 1 ||
## || Level : meta-feature level ||
## || Multimapping reads : counted ||
## || Multi-overlapping reads : not counted ||
## || Min overlapping bases : 1 ||
## || ||
## \\============================================================================//
##
## //================================= Running ==================================\\
## || ||
## || Load annotation file hg19_RefSeq_exon.txt ... ||
## || Features : 225074 ||
## || Meta-features : 25702 ||
## || Chromosomes/contigs : 52 ||
## || ||
## || Process BAM file ERR127306_chr14.bam... ||
## || Strand specific : stranded ||
## || Paired-end reads are included. ||
## || Total alignments : 400242 ||
## || Successfully assigned alignments : 171267 (42.8%) ||
## || Running time : 0.01 minutes ||
## || ||
## || Write the final count table. ||
## || Write the read assignment summary. ||
## || ||
## \\============================================================================//
You can change other options, such as the minMQS, the minimum mapping
quality score to include in the counts. To learn more about different
parameters, see the help fule for the featureCounts function.
Let’s take a peek at the output.
str(genecounts)## List of 4
## $ counts : int [1:25702, 1] 0 0 0 0 0 0 0 0 0 0 ...
## ..- attr(*, "dimnames")=List of 2
## .. ..$ : chr [1:25702] "653635" "100422834" "645520" "79501" ...
## .. ..$ : chr "ERR127306_chr14.bam"
## $ annotation:'data.frame': 25702 obs. of 6 variables:
## ..$ GeneID: int [1:25702] 653635 100422834 645520 79501 729737 100507658 100132287 100288646 729759 100131754 ...
## ..$ Chr : chr [1:25702] "chr1;chr1;chr1;chr1;chr1;chr1;chr1;chr1;chr1;chr1;chr1" "chr1" "chr1;chr1;chr1" "chr1" ...
## ..$ Start : chr [1:25702] "14362;14970;15796;16607;16858;17233;17606;17915;18268;24738;29321" "30366" "34611;35277;35721" "69091" ...
## ..$ End : chr [1:25702] "14829;15038;15947;16765;17055;17368;17742;18061;18366;24891;29370" "30503" "35174;35481;36081" "70008" ...
## ..$ Strand: chr [1:25702] "-;-;-;-;-;-;-;-;-;-;-" "+" "-;-;-" "+" ...
## ..$ Length: int [1:25702] 1769 138 1130 918 3402 793 4370 472 939 1019 ...
## $ targets : chr "ERR127306_chr14.bam"
## $ stat :'data.frame': 14 obs. of 2 variables:
## ..$ Status : chr [1:14] "Assigned" "Unassigned_Unmapped" "Unassigned_Read_Type" "Unassigned_Singleton" ...
## ..$ ERR127306_chr14.bam: int [1:14] 171267 0 0 0 0 0 0 0 0 0 ...
It looks like the counts are stored in the counts slot and the gene
annotation is stored in the annotation slot. There are also a couple
more slots for experiment metadata (targets has sample name, and
stat contains some stats about our BAM file).
Let’s take a peek at the count table, subsetted for genes on chromosome 14 (since our bam file was subsetted to reads mapping to chr 14).
chr14 <- which(grepl("chr14", genecounts$annotation$Chr))
str(chr14)## int [1:1044] 6746 6747 6748 6749 6750 6751 6752 6753 6754 6755 ...
head(genecounts$counts[chr14,])## 100132257 440153 404785 100506092 100506303 100506350
## 0 0 0 0 4 1
sum(genecounts$counts[chr14,] > 0)## [1] 499
sum(genecounts$counts[-chr14,] > 0)## [1] 0
Looks like there are 1044 genes on chromosome 14, 499 of which have non-zero expression. And as we expected, no genes on any other chromosomes have any counts.
Note that the gene IDs are RefSeq IDs. If we’d like to convert them to
another ID, such as Hugo symbols or Ensembl IDs, we can use things like
the
biomaRt
package, or annotation packages specific to the organism we are dealing
with (e.g. for human:
org.Hs.eg.db)
Let’s look at a density of expression of the chr14 genes (log-transformed).
data.frame(count = genecounts$counts[chr14,]) %>%
ggplot(aes(x = count + 1)) +
geom_density() +
scale_x_log10()We can save the count table to a text file for importing into future R
sessions for downstream analysis (such as with
DESeq2
or
edgeR.
We also include a column for gene length, since we might want to use
this to obtain quantities like RPKM.
write.table(data.frame(genecounts$annotation[,c("GeneID","Length")],
genecounts$counts),
file = "hela_counts.txt", quote = FALSE, sep = "\t", row.names = FALSE)Lastly, we’ll cleanup our directory by removing the SAM/BAM/index files we generated earlier, and the text file of counts (comment the last line if you’d like to keep the text file).
file.remove("hela.sam")
file.remove("hela_sorted.bam")
file.remove("hela_sorted.bam.bai")
file.remove("hela_counts.txt")Now we have a table that summarizes the number of reads that align to
each transcript. The is the perfect input to use for methods mentioned
above like
DESeq2
and
edgeR,
whose algorithm requires reads be in integer counts, not normalized by
library size. For visualization purposes, however, it may be helpful to
use reads that are normalized first.
Your exercise is to convert the existing count data into Counts per Million (CPM) and Reads Per Kilobase per Million mapped reads (RPKM) as defined by Bo Li et al. in this paper.
Hint: check out the cpm and rpkm functions in the edgeR package.
Format each of your answers as a numeric matrix of values.
# your code here
CPM <- "FILL_THIS_IN"
RPKM <- "FILL_THIS_IN"This next code chunk will test whether you have correctly calculated CPM and RPKM in the previous chunk. The answers are hidden as an encrypted hash, but the function will return Test passed if it matches. Note that it assumes your answers are each formatted as a numeric matrix (with one column).
test_that("Calculation of CPM:", {
local_edition(2)
expect_known_hash(round(CPM, 3), "1aaf27dc9e2203812d775a2be5963d84")
})## ── Error: Calculation of CPM: ──────────────────────────────────────────────────
## Error in `round(CPM, 3)`: non-numeric argument to mathematical function
## Backtrace:
## ▆
## 1. └─testthat::expect_known_hash(round(CPM, 3), "1aaf27dc9e2203812d775a2be5963d84")
## 2. └─testthat::quasi_label(enquo(object), arg = "object")
## 3. └─rlang::eval_bare(expr, quo_get_env(quo))
## Error:
## ! Test failed
test_that("Calculation of RPKM:", {
local_edition(2)
expect_known_hash(round(RPKM, 3), "36af8065f3dbb3b240d1f228faec5577")
})## ── Error: Calculation of RPKM: ─────────────────────────────────────────────────
## Error in `round(RPKM, 3)`: non-numeric argument to mathematical function
## Backtrace:
## ▆
## 1. └─testthat::expect_known_hash(round(RPKM, 3), "36af8065f3dbb3b240d1f228faec5577")
## 2. └─testthat::quasi_label(enquo(object), arg = "object")
## 3. └─rlang::eval_bare(expr, quo_get_env(quo))
## Error:
## ! Test failed