-
Notifications
You must be signed in to change notification settings - Fork 6
Beginner's Tutorial
In this tutorial (adapted from Tim Vaughan's Structured Coalescent tutorial), we will use the BEAST 2 package bdmm to perform a Bayesian phylogenetic analysis of an influenza data set using the multi-type birth-death model. (Note that both, the structured coalescent and the multi-type birth-death model, are tree priors implemented in BEAST2. Both of them utilize the multi-type tree structure of the MultiTypeTree package. While the structured coalescent is part of the MultiTypeTree package, the birth-death migration model has its own package bdmm.)
The data set used in this tutorial is a thinned (60 sequence) subset of the (980 sequence) data set used in the publication Vaughan et al., 2014, which in turn was assembled from publicly-available data sets provided by various authors on GenBank.
In order to proceed, ensure you have the latest version (currently 2.4) of BEAST 2 installed. To analyze the inference results you'll also need a recent versions of Tracer and an up-to-date version of Google Chrome or Mozilla Firefox.
You can easily install this package via BEAUti's package manager. To do this, follow these steps:
- Start BEAUti
- From the File menu, select Manage packages.
- Find "bdmm" in the list of packages shown, select it and then click "Install/Upgrade":

(Note the actual version of bdmm may differ from the version shown in the figure. This is normal.)
Finally, restart BEAUti. This is very important. Strange behaviour may result if you do not restart the program.
A BEAUTI template defines the basic structure and contents of your XML file. Because by default BEAUTI will construct an XML file with standard BEAST trees, rather than MultiTypeTrees, we cannot use the default template (Standard.xml). Hence, from the File menu, select Template and then choose MultiTypeBirthDeath.

Once the template is loaded, we can load in our example sequence data. In our case, this data is stored a FASTA file, the first few lines of which look like this:
> EU856841_HongKong_2005.34246575
-----------GGGATAATTCTATTAACCATGAAGACTATCATTGCTTTGAGCTACATTT
> EU856989_HongKong_2002.58356164
--CAAAAGCAGGGGATAATTCTATTAACCATGAAGACTATCATTGCTTTGAGCTACATTT...
> CY039495_HongKong_2004.5890411
------------------TTCTATTAACCATGAAGACTATCATTGCTTTGAGCTACATTC...
> EU856853_HongKong_2001.17808219
---------------------TATTAACCATGAAGACTATCATTGCTTTGAGCTACATTC...
> CY010084_NewZealand_2005.62739726
---------------------TATTAACCATGAAGACTATCATTGCTTTGAGCTACATTC...
> CY007387_NewZealand_2004.63287671
---------------------TATTAACCATGAAGACTATCATTGCTTTGAGCTACATTC...
> CY012432_NewZealand_2000.81643836
---------------------------CCATGAAGACTATCATTGCTTTGAGCTACATTT...
The lines beginning with ">" are labels for the sequences immediately following. In general, these labels have no special format, but in this file each label is a underscore-delimited triple. The first element of each triple is the GenBank accession number of the sequence, the second is the geographical region from which it was sampled, and the third is the time at which it was sampled measured in calendar years or fractions thereof. (The ellipses are not in the file, but are used here to indicate that sequence has been truncated.)
In this tutorial we will be using the influenza sequence data which is distributed with MultiTypeTree. To make this easy to find, open the File menu and select Set working dir. Then, from the submenu that appears, select MultiTypeTree.
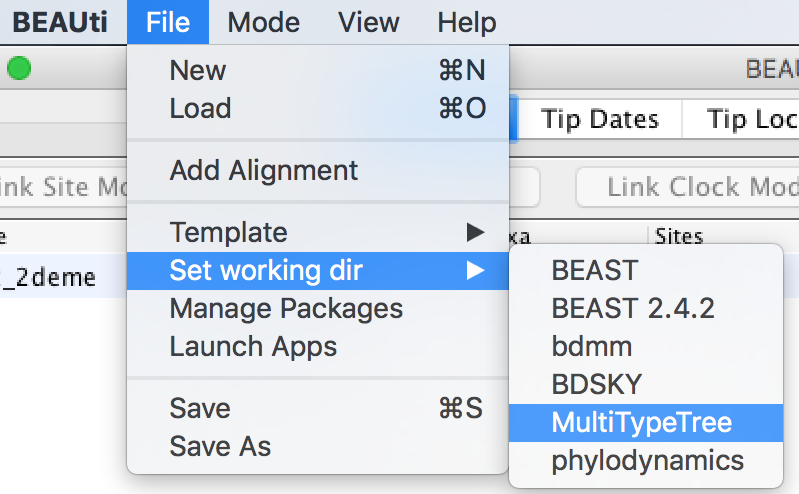
(This step is not required when loading your own data.)
To load the file, open the File menu and select Add alignment. This
will open a file selection dialog box. The example influenza sequence data
file is named h3n2_2deme.fna. Assuming you have followed the previous step to set
the working directory, this can be found in the examples/ directory shown
when the file selection dialog box loads.
Once this file is loaded, your BEAUti screen should look something like the following:

Once the data is loaded, the next step is to specify the times at which the sequences were sampled:
- Select the "Tip Dates" panel.
- Check the "use tip dates" checkbox.
- Click the "Guess" button at the top-right of the panel. This opens a dialog that allows sample times to be loaded from a file or inferred (guessed) from the sequence labels.
- Because the times are included as the last element of the underscore-delimited triple in the header, choose the "use everything" radio button and select "after last" from the drop-down menu. (Note that the underscore character is already chosen as the delimiter.)
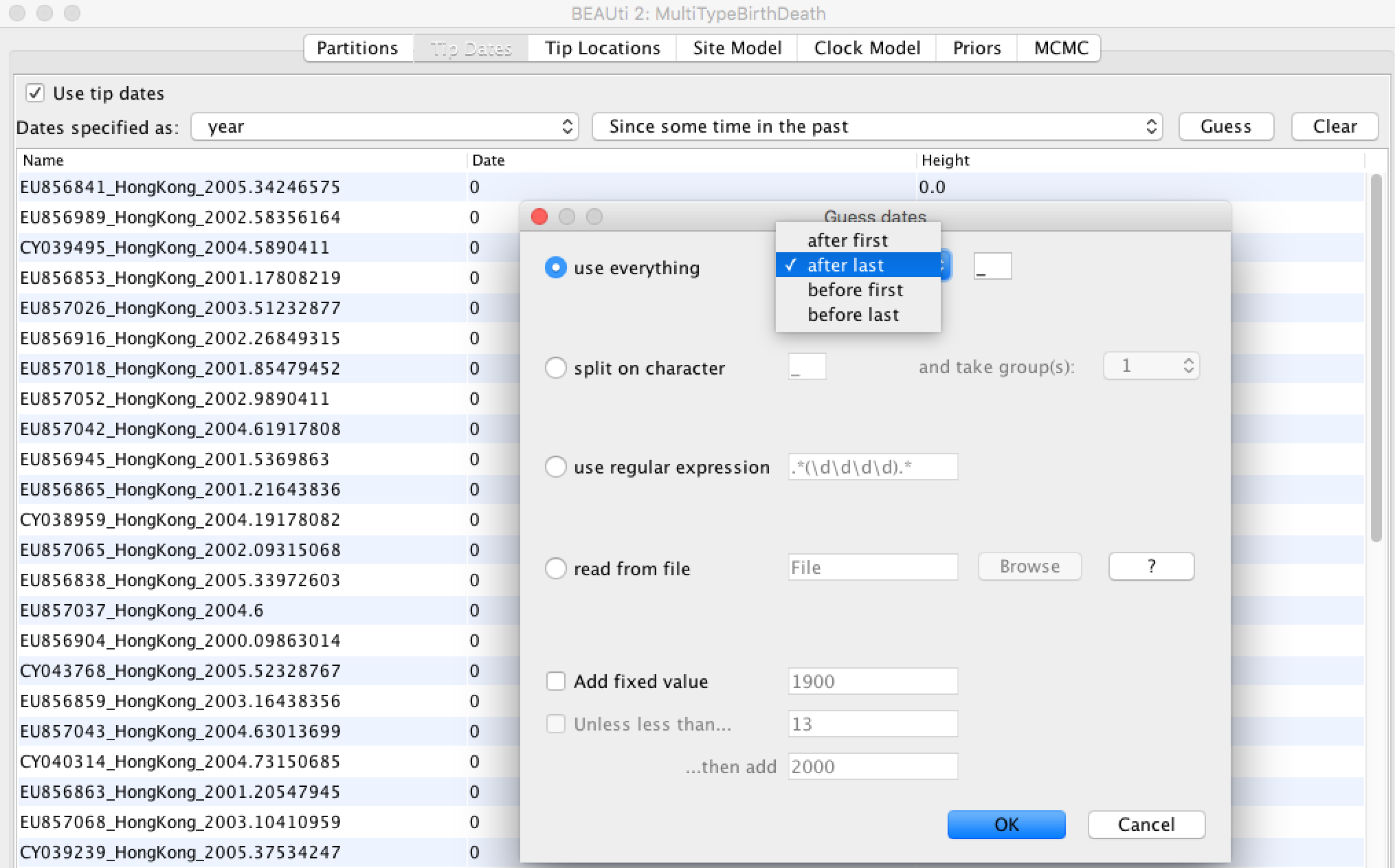
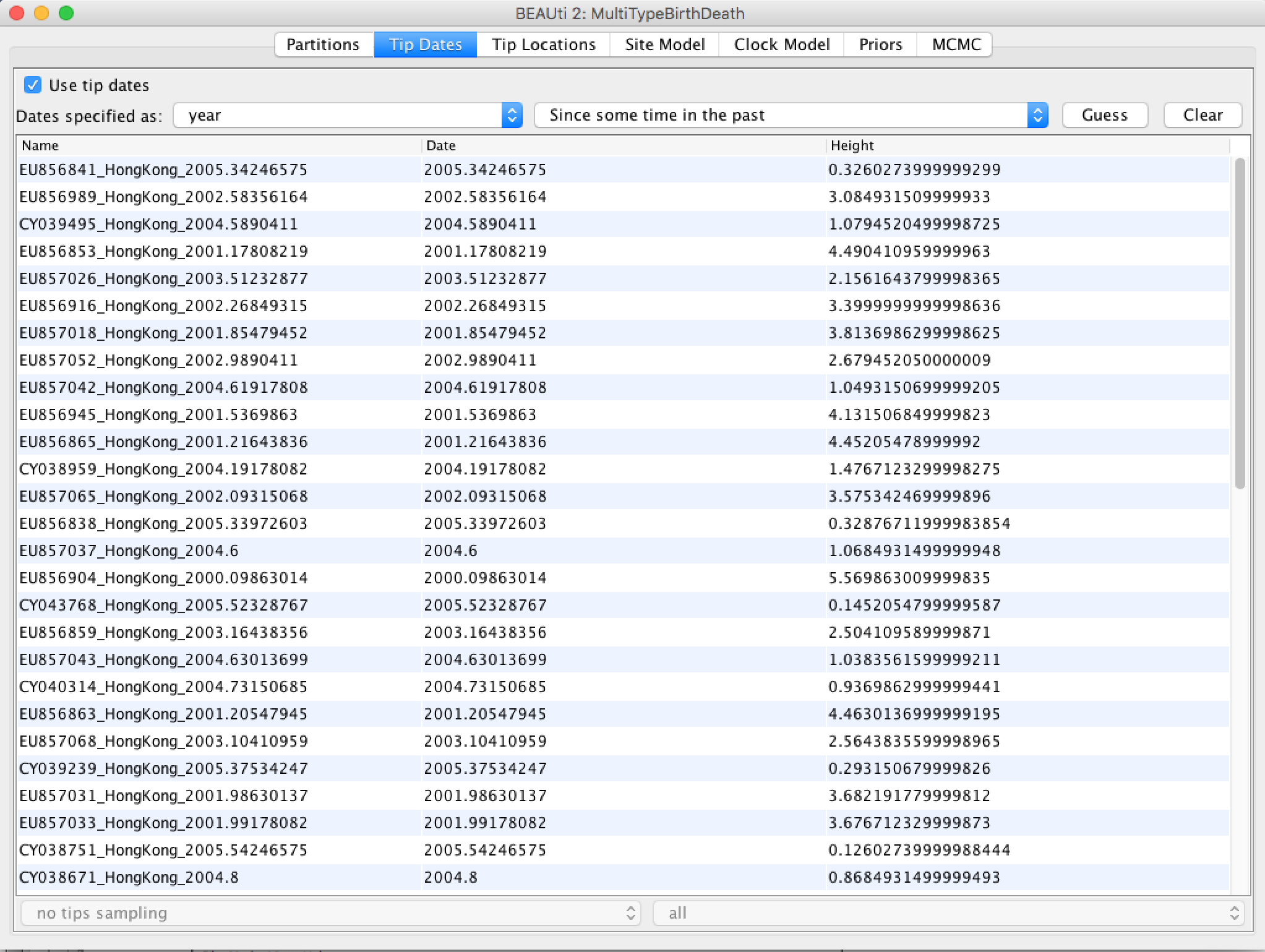
After clicking "OK" you should find that the tip date table is populated with times that match those in the sequence headers, and that the last column of the table contains "heights" (times before most recent sample) calculated from the times:
Now we've specified the sampling times, we move on to specifying the sampling locations. To do this, we follow a very similar set of steps to those we used to set the sample times:
- Select the "Tip Locations" panel. You'll find that the locations are already populated with default values.
- Click the "Guess" button at the top-right of the panel. This opens the same dialog that we saw in the previous section.
- Because the locations are included as the second element of the underscore-delimited triple in the header, choose the "split on character" radio button and select group 2 from the drop-down menu. (Note again that the underscore character is already chosen as the delimiter.)
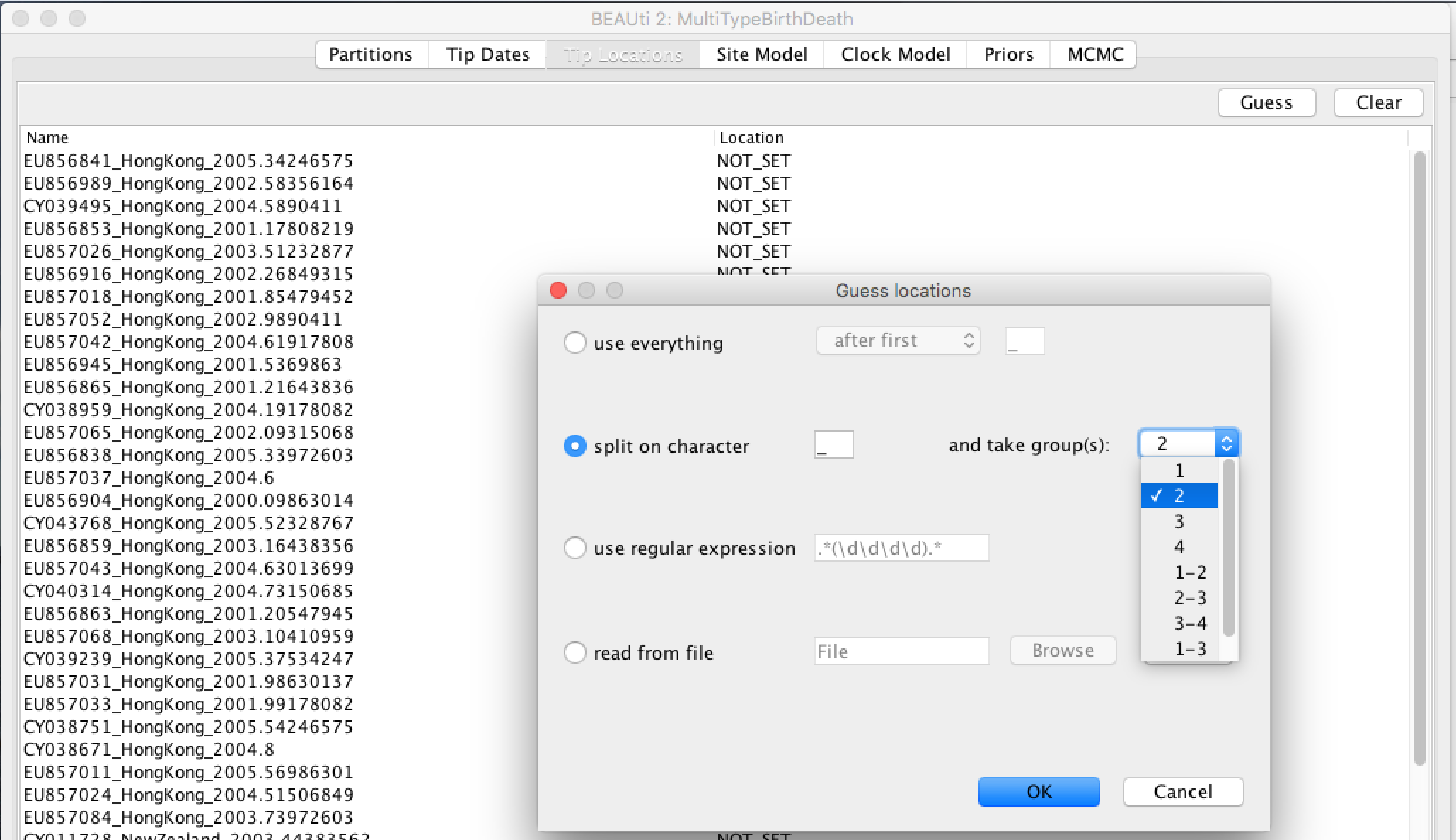
After clicking "OK" you should find that the tip location table is populated with locations that match those in the sequence headers, as follows:

For this analysis, we will use the HKY substitution model with 4 gamma categories and estimated base frequencies. To configure this in BEAUti, switch to the "Site Model" panel, change the number of gamma categories and select HKY from the drop-down menu (the default option is JC69). We also want the proportionInvariant parameter to be nonzero and estimated to account for invariant sites in our alignment.
The BEAUti panel should now look like the following:
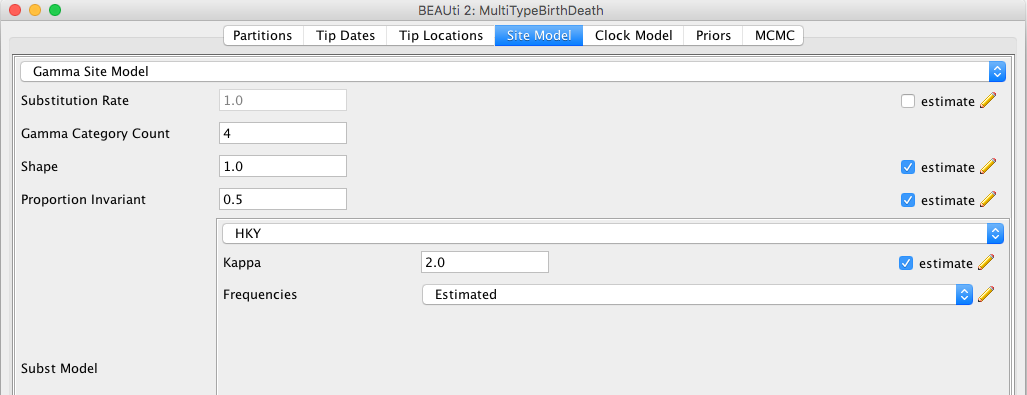
Note that the "Substitution rate" defined on this panel should be left non-estimated - we use the "Clock rate" defined in the "Clock Model" panel to determine the average per unit time rate of sequence evolution. Used this way, the "substitution rate" is therefore not actually a rate (it's actually dimensionless) but is instead a rate multiplier that in our case we fix at 1.
For this analysis we assume a strict clock. Since our alignment contains sequences sampled at different times and those times are measured in years, we must use a real clock rate expressed in units of expected substitutions per site per year. Usually the precise value is unknown and so the default behaviour of BEAUti is to assume this rate is to be estimated. We set the clock rate to 0.005 to speed up mixing. The Clock Model panel should now look like this:

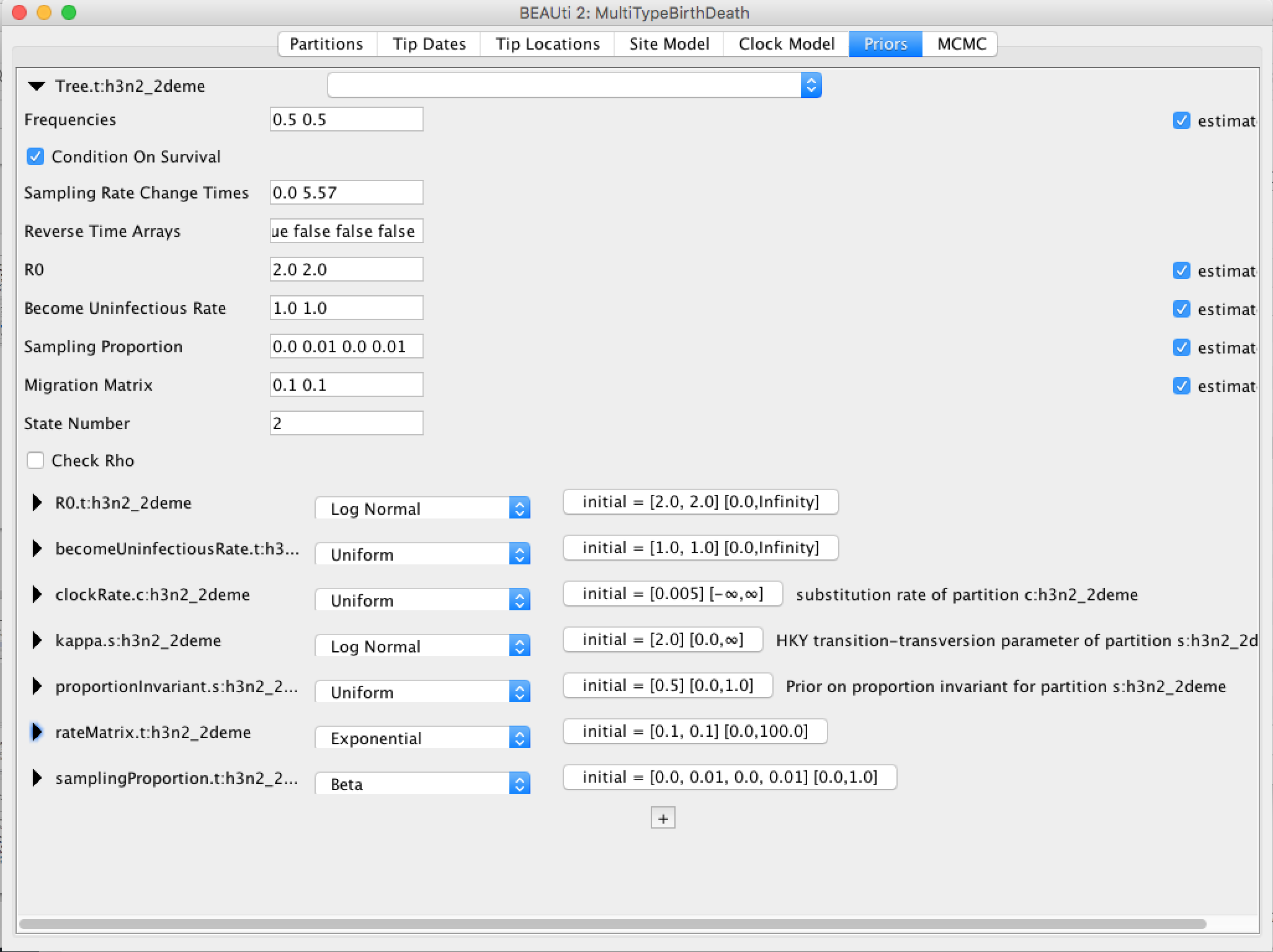
Because bdmm is a model-based prior on the (multi-type) tree distribution, setting up the "Priors" panel is a particularly important part of setting up this analysis.
Click the arrow to the left of the Tree.t:h3n2_2deme parameter to expand the tree prior:
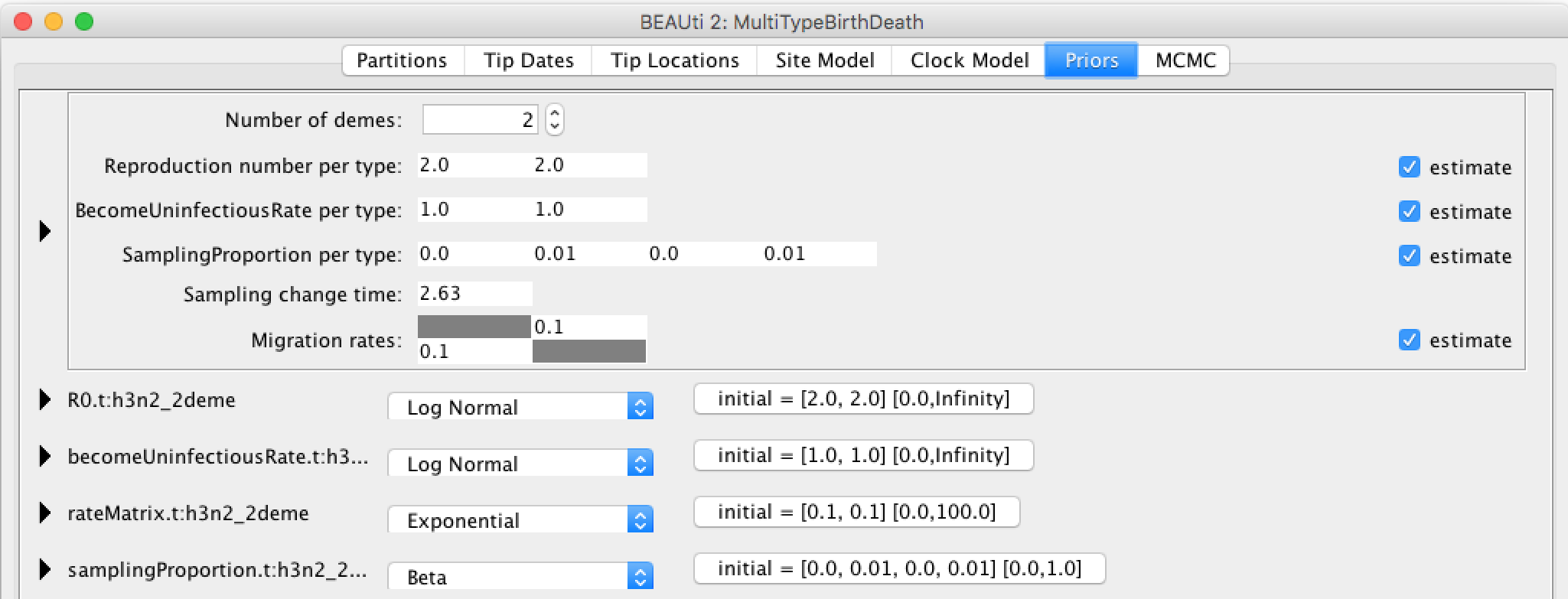
Change the time at which the sampling rate changes from 0 to something positive. This should reflect when sampling of the data set started, counting from the time of the last sample. In our case, the time between the first and last sample is 5.57 (rounded UP). Note, that the sampling proportion has 4 entries, two of which are 0. They correspond to the time interval before sampling for each of the 2 types.

Ensure the value shown in the "State Number" spinner is equal to the number of types present in your model. In this case, the default value of 2 is correct, but in general you will need to change this.
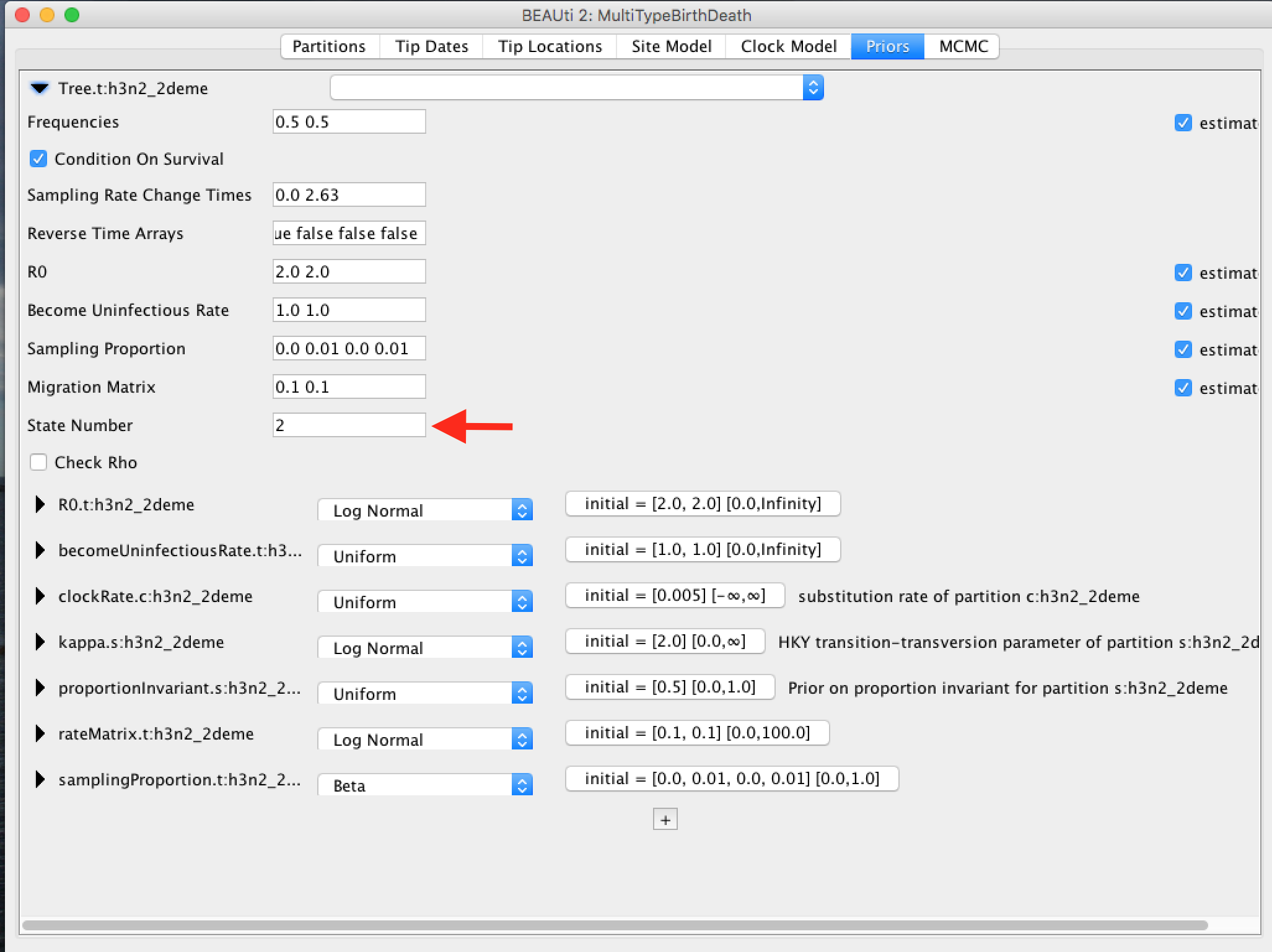
Click the arrows on the left-hand side of each parameter to alter the details of these priors. For example, we will set the rateMatrix prior distribution to Exp(1):
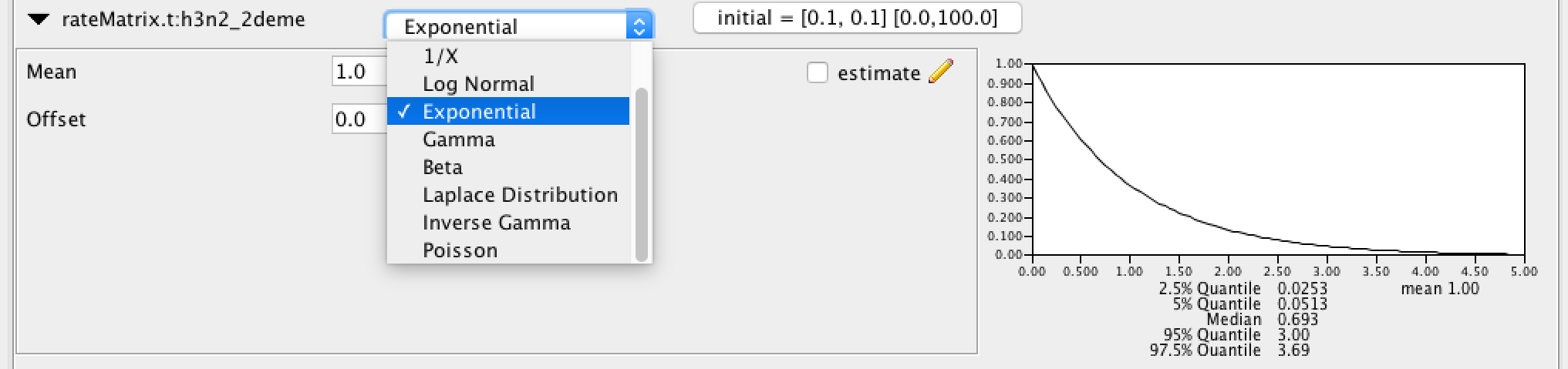
Finally, the Priors panel should look like this:
We will use the default set-up for the MCMC and save our file as usual.
To run the analysis, simply start BEAST 2 in the manner appropriate for your platform, then select the file you generated in the last section as the input file. (Refer to the documentation provided at www.beast2.org for detailed instructions.)
Since the analysis will take a while, please can download the final log files and use them to complete the tutorial.
The results of the analysis primarily consist of two parts:
- The parameter log, which is written to the file
h3n2-bdmm-v2-samplingPrior.log. - The tree log, which is written to
h3n2-bdmm-v2-samplingPrior.h3n2_2deme.trees.
In addition, the file h3n2-bdmm-v2-samplingPrior.h3n2_2deme.map.trees contains the running
estimate of the MAP tree as a function of MCMC step number, while the file
h3n2-bdmm-v2-samplingPrior.h3n2_2deme.typedNode.trees is the TreeAnnotator-compatible file
we'll use to assemble a summary tree.
We can use the program Tracer to
view the parameter log file. To do this, start Tracer and then press the "+"
button in the top-left hand corner of the window (under "Trace files"). Select
the log file for this analysis (h3n2_2deme.log) from the file selection dialog box.
The "Traces" table will then be populated with parameters and summary
statistics corresponding to our multitype birth-death analysis.
Important traces are:
-
R0.t:h3n2_2deme1andR0.t:h3n2_2deme2: These give the effective reproduction numbers for deme 1 (Hongkong) and 2 (New Zealand), respectively. -
rateMatrix.t:h3n2_2deme1rateMatrix.t:h3n2_2deme2: These give the (per lineage per year) migration rates from deme 1 to 2 and vice versa. -
Tree.t:h3n2_2deme.count_HongKong_to_NewZealand: these give the actual number of ancestral migrations from HongKong to New Zealand.
The panels tabs at the top-right of the window can be used to display one or more selected traces in various ways. For example, selecting the two R0 traces and choosing the "Marginal prob distribution" panel results in the following useful comparison between the sampled population size marginal posterior distributions:
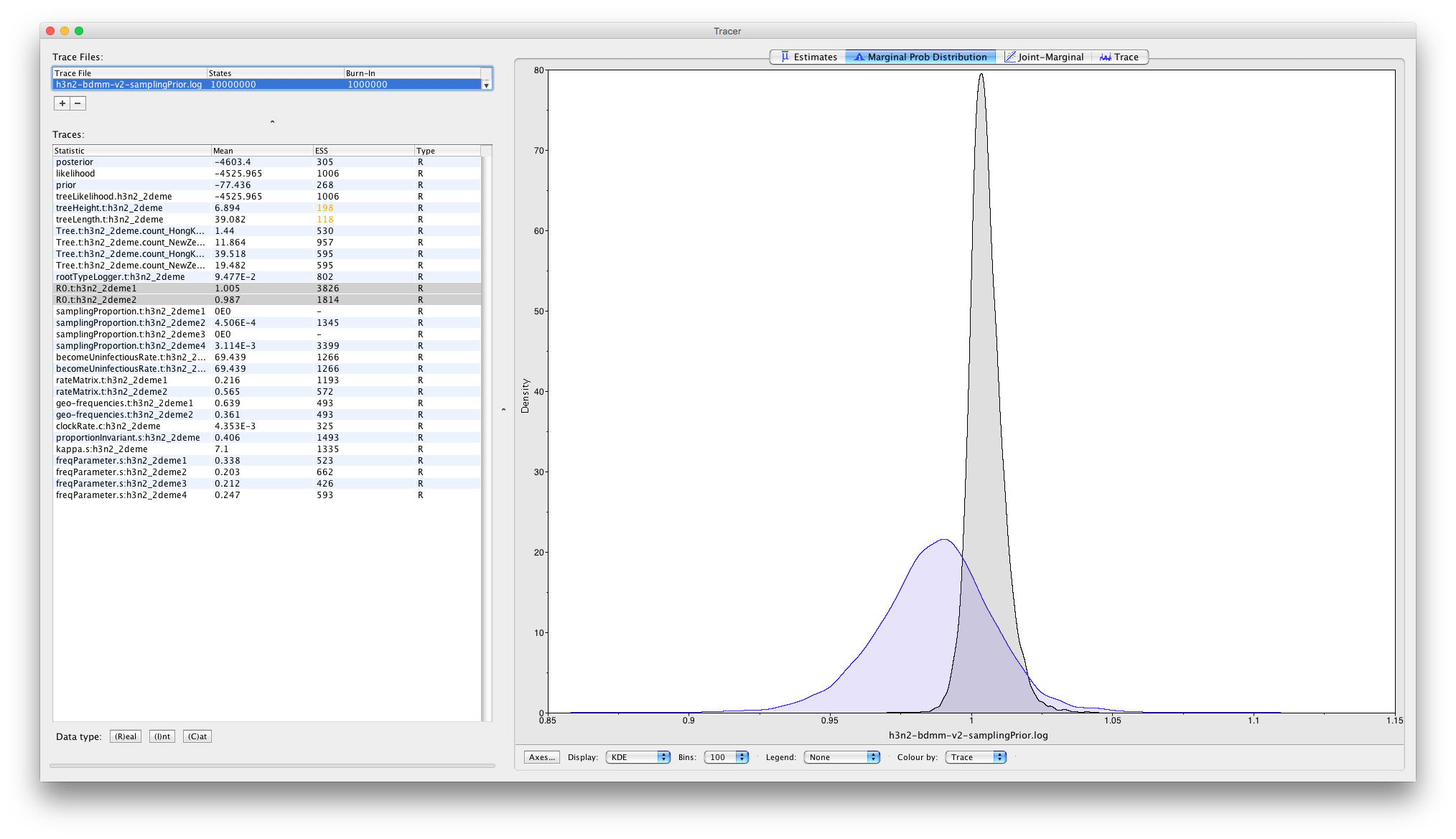
Note that some of the ESS values are still less than 200 - the arbitrary threshold for acceptability. If this analysis were part of a serious study you would want to run the chain for another few million iterations to improve these. (In BEAST 2, analyses can be resumed - the samples you've already acquired will not be wasted.) For the purposes of this tutorial, however, these values are acceptable.
The popular phylogenetic tree visualizer FigTree can be used to visualize the sampled trees. Be warned, however, that FigTree currently takes an extremely long time to load even relatively small (a few megabyte) MultiTypeTree logs.
For this reasons we suggest using IcyTree to view tree log files and maybe switching to FigTree to visualize summary trees as discussed in the next section. (Also, IcyTree can be used to export individual trees from a large log file for subsequent viewing using FigTree.) IcyTree is a tree viewer that runs in a web browser. It runs best under recent versions of Google Chrome and Mozilla Firefox (in that order).
To view MultiTypeTree log files using IcyTree, simply navigate to the IcyTree web page, select "Load from file" from the "File" menu, then select one of the tree log files using the file selection dialog. Once the file is loaded you will see the first tree it contains. In order to select a different tree, move the mouse pointer over the box in the lower-left corner of the window. This box will expand to a small dialog containing buttons allowing you to navigate between trees. The '<' and '>' buttons move in steps of 1 tree, while '<<' and '>>' move 10% of the tree file per click. You can also directly enter the index of a tree. (Note that there are keyboard shortcuts for almost all commands in IcyTree and that these can be found by selecting "Keyboard shortcuts" from the "Help" menu.)
Initially the trees edges will be uncoloured. To colour the edges according to the edge type, open the "Style" menu, navigate to the "Colour edges by" submenu and select "type". A legend and axis can be added by choosing "Display legend" and "Axis > Age" from the same menu.
The following shows the final tree of h3n2-bdmm-v2-samplingPrior.h3n2_2deme.map.trees in IcyTree, which
represents our sampled estimate of the MAP multi-type tree:

While IcyTree is useful for rapidly visualizing the results of an analysis, it is not nearly as feature-rich as FigTree and not as capable for producing publication-quality graphics. Happily, however, IcyTree can extract single trees from larger log files. Simply navigate to the desired tree, open the "File" menu, choose the "Export tree as" submenu and select "NEXUS file". (It is important to select "NEXUS" instead of "Newick" as the Newick format does not support the annotations that MultiTypeTree uses to mark the edge types.
While it is tempting to view the MAP tree shown above as the primary result of the phylogenetic side of our analysis it is very important to remember that this is only a point estimate and says nothing about the uncertainty present in the result. This is an important drawback, as we have done a full Bayesian analysis and have access to a large number of samples from the full posterior in the tree log files. The MAP tree discards almost all of this information.
We can make better use of our raw analysis results by using the TreeAnnotator
program which is distributed with BEAST to analyze the
typedNode trees which were produced by our MCMC run. To do this,
simply load TreeAnnotator and select the typedNode tree file as
the input file and h3n2-bdmm-v2-samplingPrior.h3n2_2deme.summary.trees as the output file. Select "Mean
heights" from the "Node heights" menu and set the burn-in percentage to 10:

Pressing the "Run" button will now produce an annotated summary tree.
To visualize this tree, open IcyTree once more (maybe open it in a new browser
tab), choose File->Open, then select the file
h3n2_2deme.h3n2_2deme.summary.tree using the file selection dialog. Follow
the instructions provided for the MAP tree above to colour the tree by the
"type" attribute and add the legend and time axis. In addition, open the Style
menu and from the "Node height error bars" sub-menu select "height_95%_HPD" to
add error bars to the internal node heights. Also, open the Style menu and from
the "Edge opacity" sub-menu select "type.prob". This will cause the edges to
become increasingly transparent as the posterior probability for the displayed
colour decreases.
Once these style preferences have been set, you should see something similar to the following:

Here we have a full consensus tree annotated by the locations at coalescence nodes and showing node height uncertainty, with the widths of the edges representing how certain we can be of the location estimate at each point on the tree. This is a much more comprehensive summary of the phylogenetic side of our analysis.
One thing to pay attention to here is that the most probable root location is
given by the summary tree to be Hong Kong (under our model which assumes that
only Hong Kong and New Zealand exist). By hovering the mouse cursor over the
tiny edge above the root will bring up a table in which posterior probability
of the displayed root location (type.prob) can be seen to be approximately
90%. The analysis therefore strongly supports a Hong Kong origin over a New
Zealand origin.