diff --git a/docs/project/results.md b/docs/project/results.md
index 4e61c44..94758c2 100644
--- a/docs/project/results.md
+++ b/docs/project/results.md
@@ -51,7 +51,7 @@ In this setup, one half of the RNAP is fused to ubiquitin, while the other half
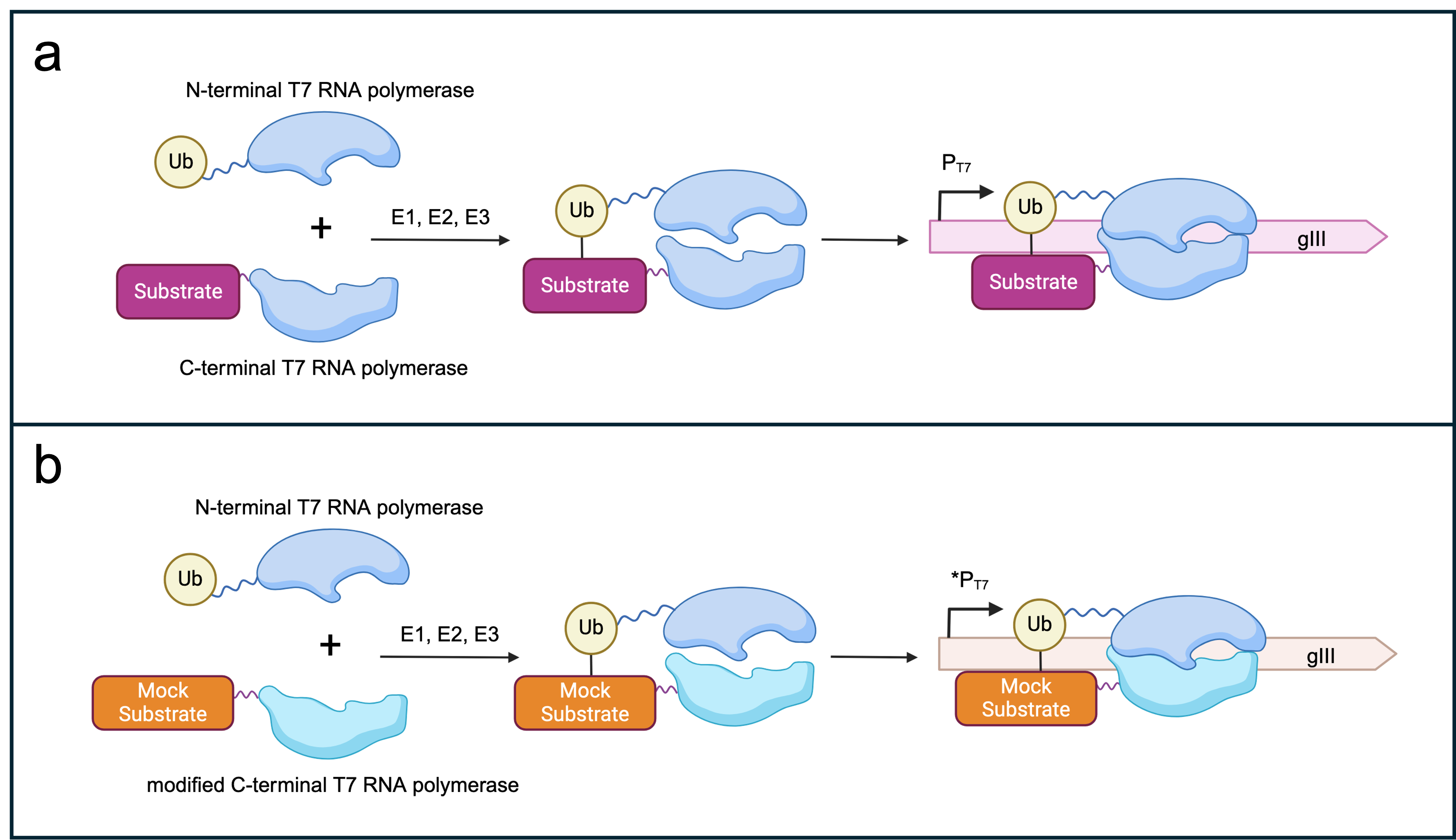
- Figure 3: Selection logic for SIAH1/2-dependent _gIII_ expression. (a) Split T7 RNAP subunits fused to ubiquitin or a canonical substrate of SIAH1/2. The presence of E1, E2 and E3 (SIAH1/2) should lead to the assembly of the T7 RNAP subunits and thereby _gIII_ transcription under the control of a T7 promoter. (b) Potential off-target effects of the evolved SIAH1/2 could be selected against by punishing spurious ubiquitination of a mock substrate by E3 ligase. In a new AP1neg plasmid, a mutated version of the C-term RNAP subunit that recognises a modified T7 promoter sequence [1] is fused to a mock substrate. A non-functional _gIII_ (here, mock _gIII_) is placed under the control of the modified T7 promoter. Recognition and subsequent ubiquitination of the mock substrate by the evolved E3 ligase leads to the expression of mock _gIII_. Consequently, the phage offspring are not able to propagate further. Figure created with BioRender.com.
+ Figure 3: Selection logic for SIAH1/2-dependent gIII expression. (a) Split T7 RNAP subunits fused to ubiquitin or a canonical substrate of SIAH1/2. The presence of E1, E2 and E3 (SIAH1/2) should lead to the assembly of the T7 RNAP subunits and thereby gIII transcription under the control of a T7 promoter. (b) Potential off-target effects of the evolved SIAH1/2 could be selected against by punishing spurious ubiquitination of a mock substrate by E3 ligase. In a new AP1neg plasmid, a mutated version of the C-term RNAP subunit that recognises a modified T7 promoter sequence [1] is fused to a mock substrate. A non-functional gIII (here, mock gIII) is placed under the control of the modified T7 promoter. Recognition and subsequent ubiquitination of the mock substrate by the evolved E3 ligase leads to the expression of mock gIII. Consequently, the phage offspring are not able to propagate further. Figure created with BioRender.com.
@@ -64,7 +64,7 @@ We plan to run this system in a bioreactor to create a continuous evolutionary e
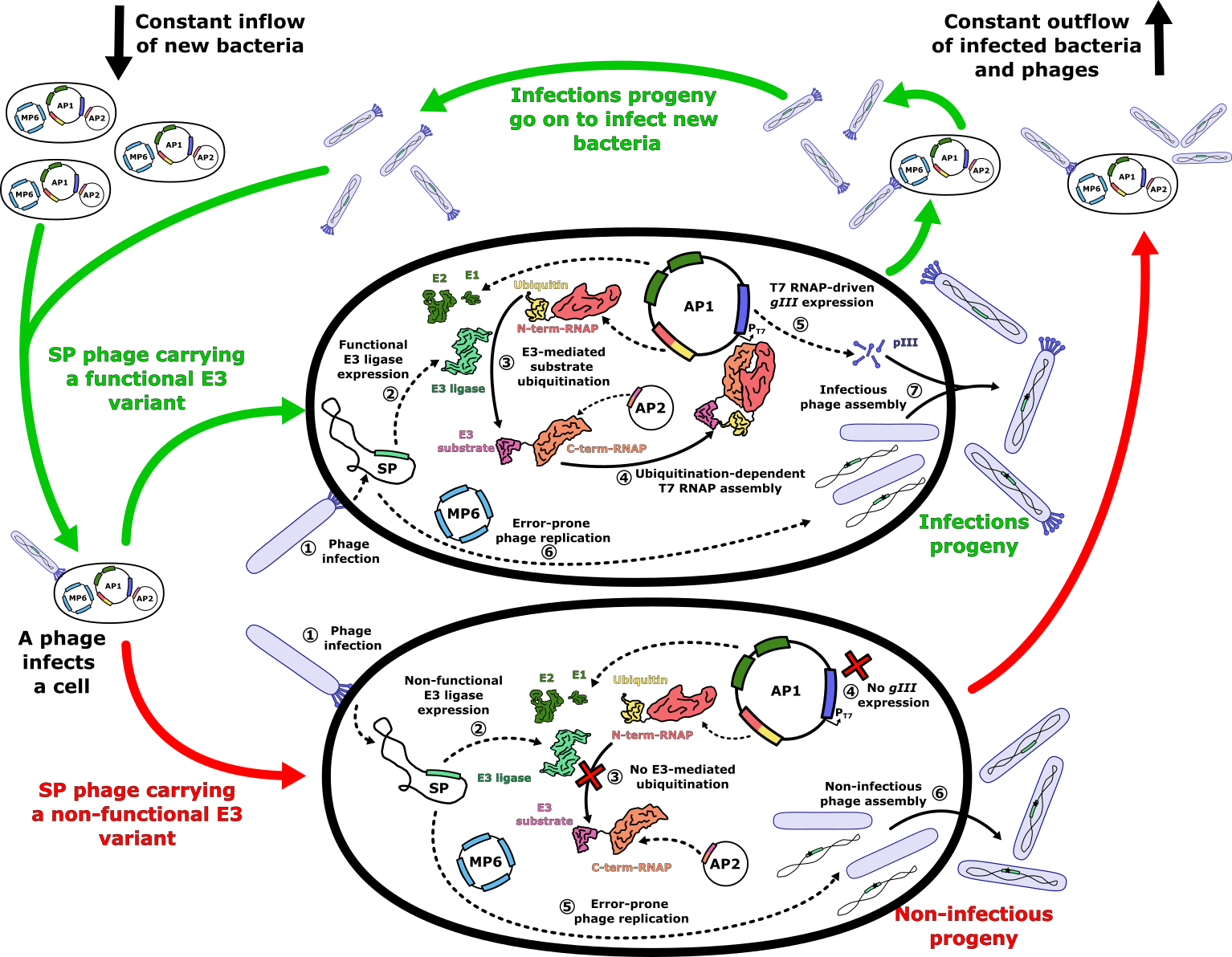
- Figure 4: E3 ligase PACE evolutionary system. The PACE system operates within a lagoon with a constant inflow of new host cells and an outflow of phages and infected host cells. Upon infection of a host cell with a selection phage carrying a functional E3 ligase variant (green arrows), the E3 ligase ubiquitinates its target, which is fused to the C-terminal subunit of a split T7 RNA polymerase (RNAP), using ubiquitin fused to the N-terminal subunit of the split RNAP. The ubiquitin-mediated proximity of the split RNAP subunits assembles a functional T7 RNAP, which transcribes the _gIII_ gene required for the assembly of infectious progeny. In the case of a non-functional E3 variant (red arrows), the lack of assembled T7 RNAP prevents _gIII_ expression, resulting in non-infectious phage progeny. During phage genome replication, the mutation plasmid MP6 increases the mutation rate, generating new E3 variants in the process. Infectious progeny carrying new E3 variants then go on to infect fresh host cells, repeating the cycle.
+ Figure 4: E3 ligase PACE evolutionary system. The PACE system operates within a lagoon with a constant inflow of new host cells and an outflow of phages and infected host cells. Upon infection of a host cell with a selection phage carrying a functional E3 ligase variant (green arrows), the E3 ligase ubiquitinates its target, which is fused to the C-terminal subunit of a split T7 RNA polymerase (RNAP), using ubiquitin fused to the N-terminal subunit of the split RNAP. The ubiquitin-mediated proximity of the split RNAP subunits assembles a functional T7 RNAP, which transcribes the gIII gene required for the assembly of infectious progeny. In the case of a non-functional E3 variant (red arrows), the lack of assembled T7 RNAP prevents gIII expression, resulting in non-infectious phage progeny. During phage genome replication, the mutation plasmid MP6 increases the mutation rate, generating new E3 variants in the process. Infectious progeny carrying new E3 variants then go on to infect fresh host cells, repeating the cycle.